A “significant breakthrough” in color switching for nanocrystals that unlocks exciting possibilities for a simple, energy-efficient display design and for tunable light sources for many technologies has been achieved by researchers at the Hebrew University of Jerusalem (HU).
Colored light and its tunability are the basis of many essential modern-day technologies from lighting and displays to fast optical fiber-communication networks.
The discovery also has potential applications for sensitive sensors of various substances, including biological and neuroscience uses and advancements in quantum-communication technologies. This nanomaterial breakthrough holds the promise of inspiring exciting innovations in the future, the team said.
They published their findings in the prestigious journal Nature Materials under the title “Electric field induced color switching in colloidal quantum dot molecules at room temperature.”
While nanocrystals offer color tunability and are used in various technologies, achieving a variety of colors requires using different nanocrystals for each color and dynamic switching between colors has not been possible.
Overcoming nanocrystal tech barriers
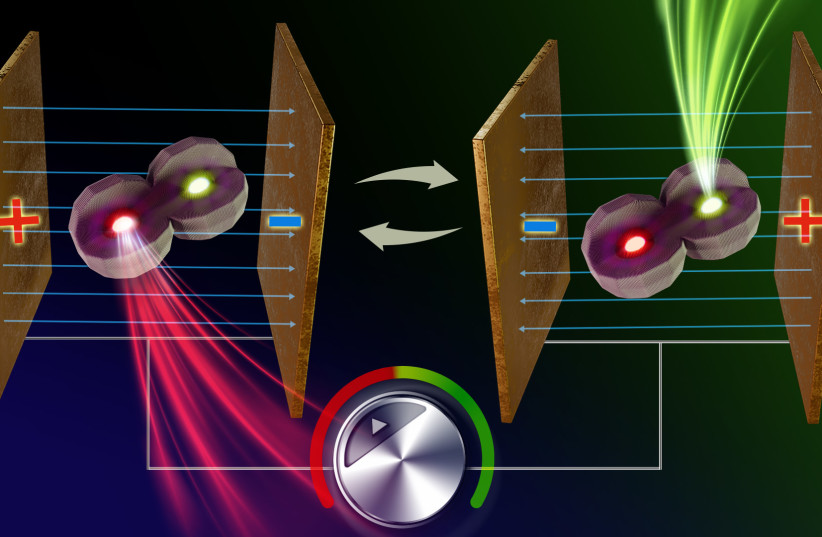
A team at HU’s Institute of Chemistry and the Center for Nanoscience and Nanotechnology including graduate student Yonatan Ossia with seven other members and led by Prof. Uri Banin, have now come up with an innovative solution to this problem. By developing a system of an “artificial molecule” made of two coupled semiconductor nanocrystals that emit light in two different colors, fast and instantaneous color switching was achieved.
Banin said that “our research is a big leap forward in nanomaterials for optoelectronics. This is an important step in our exposition of the idea of “nanocrystal chemistry” launched just a few years ago in our research group in which nanocrystals are building blocks of artificial molecules with exciting new functionalities.
Being able to switch colors so quickly and efficiently on the nanoscale as we have achieved has enormous possibilities. It could revolutionize advanced displays and create color-switchable single photon sources.”
When taking color-emitting semiconductors to the nanoscale (a nano is one-billionth of a meter, 100,000 times smaller than a human hair), an effect called quantum confinement comes into play. Changing the size of the nanocrystal modifies the color of the emitted light, so bright light sources can be obtained covering the entire visible spectrum.
Because of the unique color tunability of such nanocrystals and their easy fabrication and manipulation using wet chemistry, they are already widely used in high-quality commercial displays, giving them excellent color quality along with significant energy-saving characteristics.
But until now, achieving different colors such as needed for the different RGB pixels (composed of red, green and blue subpixels that light up at different intensities to create different colors and use in electronic displays like TVs, computer monitors, lighting, and digital cameras) required the use of different nanocrystals for each specific color. However, dynamic switching between the different colors was not possible.
Although color tuning of single colloidal nanocrystals which behave as “artificial atoms” has been studied and implemented in prototype optoelectronic devices, changing colors actively has been challenging because of the diminished brightness inherently accompanying the effect that produced only a slight shift of the color.
The research team overcame this limitation, by creating a novel molecule with two emission centers in which an electric field can tune the relative emission from each center, changing the color, yet, without losing brightness.
The artificial molecule can be made such that one of its constituent nanocrystals is tuned to emit “green” light, while the other “red” light. The emission of this new dual color emitting artificial molecule is sensitive to external voltage inducing an electric field – one polarity of the field induces emission of light from the “red” center, and switching the field to the other polarity, the color emission is switched instantaneously to “green”, and vice versa.
This color-switching phenomenon is reversible and immediate, as it does not include any structural motion of the molecule.
