An 11-year-old Palestinian boy and a female cousin of his have been found by Jerusalem researchers to be able to eat very large amounts of red chili peppers – which contain capsaicin – and not feel any pain at all from it. But this “pepper boy,” who has been eating large amounts of chili peppers since the age of three, does feel other painful stimuli, basically undermining the significance of this study and others on mice.
Capsaicin, the chemical that makes foods spicy and is released as a fine spray when you bite into foods that contain it, triggers heat receptors in the skin, tricking the nervous system into thinking you’re overheating. In response, the brain cranks up all of your body’s cooling mechanisms. It is used to help relieve a certain type of shooting or burning pain in the nerves (neuralgia) and can also relieve minor pain associated with rheumatoid arthritis or muscle sprains and strains.
It also uniquely increases energy expenditure and boosts testosterone levels, so eating a low-calorie diet makes capsaicin popular among bodybuilders, especially during pre-contest training, when they try to shed as much body fat as possible without losing any muscle mass.
As to how much capsaicin would cause toxicity and lethal side effects in humans, it is estimated to be around 12 to 13 grams for a person weighing 68 kilos. Most capsaicin supplements on the market contain cayenne pepper as the main active ingredient, as it tends to be high in the chemical, which was first isolated from chili peppers in crystalline form in 1878. Soon after, it was discovered that capsaicin caused a burning sensation in the mucous membranes. It also raised the amount of gastric acid and stimulated the nerve endings in the skin.
Pain afflicts at least 1.5 billion people worldwide, and despite the availability of various painkilling drugs, not all forms of pain can be treated effectively. Moreover, pain medications can have side effects such as dependence and tolerance, especially in the case of morphine and other opioids.
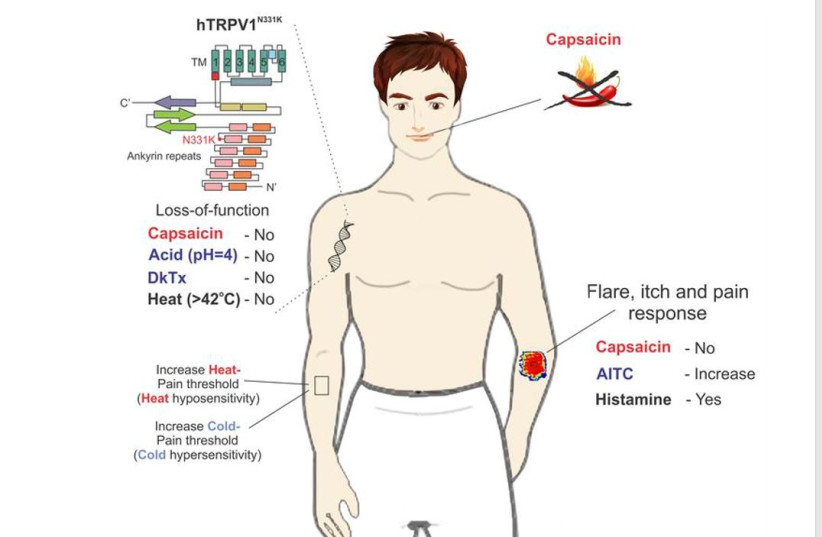
Study provides direct evidence of pain-related functional changes linked to TRPV1
The study, the authors wrote, “provides what to our knowledge is the first direct evidence for pain-related functional changes linked to TRPV1 in humans, which should be considered in the development of TRPV1 antagonists as novel therapeutic pain-relieving agents.” (TRPV1 is the transient receptor potential vanilloid 1 channel.) The two relatives have a genetic mutation that caused this.
The study was conducted by Baruch Minke, Rachel Zaguri, Channa Maayan, Orly Elpeleg, Shaya Lev, Elyad Davidson, Maximilian Peters, Shlomit Kfir-Erenfeld, Esther Berger, Shifa Ghazalin and Alexander Binshtok and published in the Journal of Clinical Investigation under the title “Nociception and pain in humans lacking a functional TRPV1 channel.”
The researchers work at the medical neurobiology department at Faculty of Medicine and the Edmond and Lily Safra Center for Brain Sciences (ELSC) of the Hebrew University of Jerusalem; the pediatric neurology unit, pediatric department at Hadassah University Hospital on Mount Scopus in Jerusalem; the human genetics department, the pain relief unit in the anesthesiology and critical care medicine department and the department bone-marrow transplantation and cancer immunology department at Hadassah University Medical Center in Ein Kerem; and the pathology department at Wolfson Medical Center in Holon.
The study provides direct evidence in humans for pain-related functional changes linked to TRPV1, which is associated with noxious heat detection and inflammatory pain. Reports of adverse effects in human trials have hindered extensive efforts in the clinical development of TRPV1 antagonists as pain relievers.
The team studied the cousins who carry a homozygous mutation in TRPV1, rendering the channel nonfunctional. Parental consanguinity has been associated with an increased risk of autosomal recessive disorders and congenital anomalies in the offspring (52). Since the affected individuals (A1 and A2) are the descendants of a consanguineous marriage, biochemical and functional assays were used to analyze the mutant channel. To identify possible phenotypes of the affected individuals, they performed psychophysical and medical examinations.
The cousins were insensitive to the application of capsaicin to the mouth and skin and did not demonstrate aversive behavior towards it. Parental consanguinity has been linked with an increased risk of autosomal recessive disorders and congenital anomalies in the offspring.
The researchers said their study provides “direct evidence in humans to sense [that] noxious stimuli is crucial for protecting organisms from tissue damage. The discovery of the capsaicin and TRPV1 has advanced the molecular understanding of noxious heat sensation and inflammatory pain.
“To our knowledge, only two studies that utilized the genetic approach have been reported for the hTRPV1 gene,” they said. “First, is a study describing an individual carrying mutations in the second intron of hTRPV1, resulting in a reduction of approximately 65% of TRPV1 protein levels. These mutations caused insensitivity to capsaicin, hypersensitivity to garlic extract applied to the mouth, and similar, but slightly prolonged, response latencies to noxious heat compared with healthy volunteers. The second involves a study of patients with cystinosis, who harbored a deletion in the CTNS gene.”
