People with long-term paralysis might regain the ability to walk after Israeli scientists successfully engineered the first 3D human spinal cord tissue. The results were published in a groundbreaking peer-reviewed study published in the journal Advanced Science on Monday morning.
The study was conducted by researchers from the Sagol Center for Regenerative Biotechnology at Tel Aviv University, headed by Prof. Tal Dvir. He was joined by researchers from the Shmunis School of Biomedicine and Cancer Research, and the Department of Biomedical Engineering at TAU. The team at Dvir’s lab includes PhD student Lior Wertheim, Dr. Reuven Edri and Dr. Yona Goldshmit.
Why haven’t we been able to heal spinal cord injuries?
Paralysis can occur after a spinal cord injury, which can refer to damage sustained to any part of the spinal cord or the nerves at the end of the spinal canal. These injuries can cause permanent changes in strength, sensation and other bodily functions, and in severe cases, can lead to long-term paralysis, for which there is currently no cure.
Despite many prior attempts having been made worldwide to promote natural or intervened regeneration at the site of the injury, there has been minimal success.
Many existing experimental or investigated methods rely on the transplantation of different cells or biomaterials into the site of the injury. Two issues jeopardize the success of the treatment, however: the immune response to the transplanted cells causing them to be rejected, and the implantation of dissociated cells that fail to form into a functional network.
Therefore, the research team hypothesized that mimicking embryonic development by applying a specific spinal cord motor neuron differentiation protocol in a 3D dynamic environment would provide cells with signals for appropriate regenerative tissue formation, healing the site and lowering the risk of rejection.
Furthermore, they theorized, assembling a functional neuron network prior to implantation would increase the chances of functional engraftment, in which it integrates well into the host body.

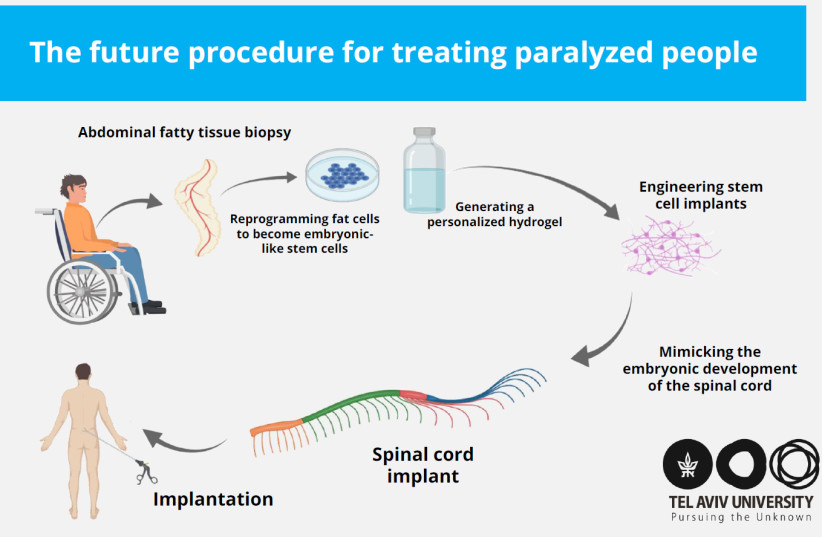
THE PROCEDURE developed by the research team would involve taking a small fatty tissue biopsy from the patient and separating it into the cells and the extracellular biomaterial.
The cells would then be reprogrammed to become patient-specific induced pluripotent stem cells (iPSCs) – a cell type used in regenerative medicine that can propagate indefinitely and can be used to replace cells lost to damage or disease.
Meanwhile, the biomaterial undergoes a process to turn it into a personalized hydrogel, into which the embryonic-like iPSC cells are then encapsulated, allowing them to differentiate into a 3D spinal cord network.
Not only does the biomaterial turned hydrogel support the cells, the study explained, but it also constantly adapts and develops, thereby providing a dynamic inductive microenvironment that allows for the assembly and maturation of a functional spinal cord implant.
Following the successful mimicking of embryonic spinal cord development and the engineering of functional tissue implants, the researchers moved on to testing the therapeutic potential of the 3D spinal cord network, choosing to use mice as the testing model.
The mice were divided into two groups - those who had been recently paralyzed (acute), and those who had been paralyzed for at least a year in human terms (chronic).
The mice with acute paralyzation regained the ability to walk within the space of three months after the insertion of the implant, showing significant gains over mice with acute paralysis that had been left untreated.
While the untreated mice did regain partial motor function over time, they showed worse coordination, and a greatly decreased ability to place pressure on the injured foot, among other issues, than those that underwent the implantation of the lab-grown spinal cord.
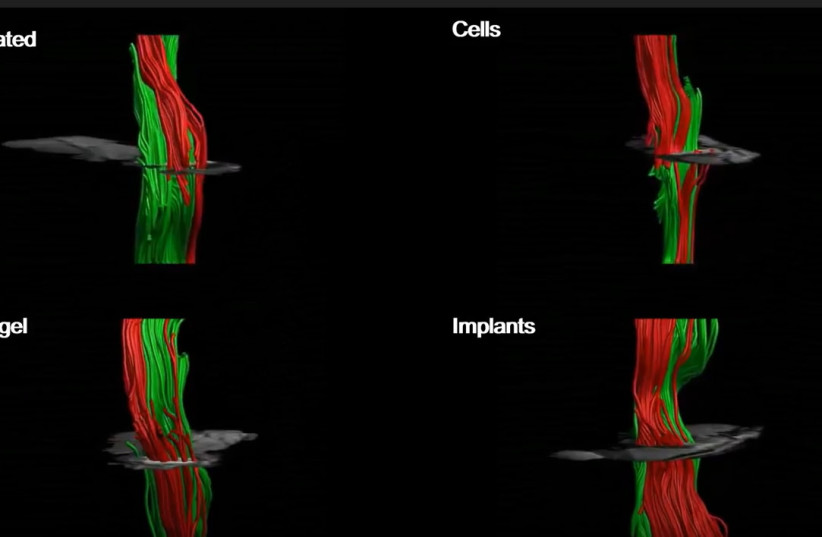
Following the success observed in the acute phase of injury, the research team moved on to testing the same theory in the mice with chronic paralyzation, a more clinically relevant model due to the extent of permanent damage to the spinal cord still being unclear during the acute phase of paralysis.
Six weeks after implanting the artificial spinal cord into the mice with chronic paralyzation, the animals showed significant improvement, indicating that the implant had successfully been integrated into the body. Overall, 80% of the mice in the test group regained the ability to walk.
“The model animals underwent a rapid rehabilitation process, at the end of which they could walk quite well,” Dvir explained. “This is the first instance in the world in which implanted engineered human tissues have generated recovery in an animal model for long-term chronic paralysis, which is the most relevant model for paralysis treatments in humans.”

FOLLOWING THE success seen in the lab trials and the results observed in the mice post-implant, the researchers hope to progress to clinical trials in humans within the next few years. They have already held talks with the FDA regarding the preclinical program.
“Since we are proposing an advanced technology in regenerative medicine, and since at present there is no alternative for paralyzed patients, we have good reason to expect relatively rapid approval of our technology,” he explained.
Based on this revolutionary organ-engineering technology, Dvir teamed up with industry partners to establish Matricelf in 2019. The company applies his approach in their work with the aim of making spinal cord implant treatments commercially available.
How could this impact the field of medicine?
While the study specifically focused on injured spinal cords, the researchers hope that in the future the same technology could be applied and used to treat a variety of different diseases and injuries such as Parkinson’s, brain trauma, myocardial infarction and age-related macular degeneration, all of which they are currently researching through this technology.
“There are millions of people around the world who are paralyzed due to spinal injury, and there is still no effective treatment for their condition,” Dvir said.
“Individuals injured at a very young age are destined to sit in a wheelchair for the rest of their lives, bearing all the social, financial and health-related costs of paralysis," he said. "Our goal is to produce personalized spinal cord implants for every paralyzed person, enabling regeneration of the damaged tissue with no risk of rejection.”
